
The chemical power source that will be made in this master class has quite a significant power to obtain with it a voltage capable of powering 220 V network devices.
You've probably seen articles on the Internet where electricity is obtained from a lemon by inserting two electrodes made of different metals into it. This battery will be built according to the same principles, only on a larger scale.
Let's just go not along the path of increasing the sections of elements, but along the path of increasing the area of the electrodes, which should give greater battery current, and therefore the power of the entire installation.
Water and baking soda diluted in it will be used as an electrolyte.
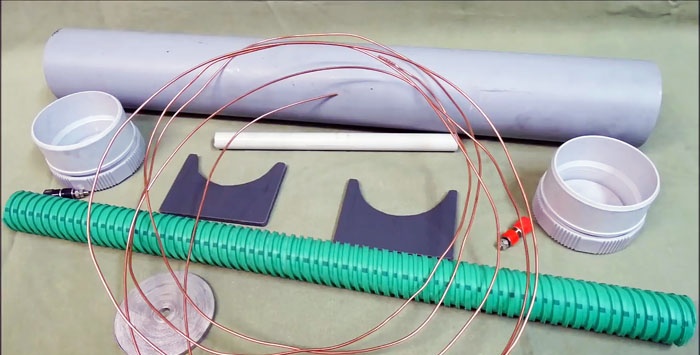
Will need
- PVC sewer pipe, length 1-1.2 m.
- Two PVC plugs.
- Copper wire.
- Galvanized strip.
- A piece of corrugated pipe.
- Thin PVC tube.
- A couple of pieces of plastic for stands.
- There are two terminals.
We make a water-powered battery
We need to assemble a sealed vessel from a PVC pipe - this will be the body of our battery. I decided to insert screw caps at the ends so that they could be unscrewed at any time. Use a gas burner to heat the edge of the pipe.

We insert the plug.

The result is this neat edge with a thread at the end.

We glue pieces of thin pipe into the caps of the plugs. There is no need to make a hole in them. These segments will center the internal element and are needed only as fasteners. We use epoxy resin based glue.

The entire battery will be positioned horizontally; to do this, we glue special legs on both sides.

It's time to make the electrode element itself. We take a tube with a serpentine texture and first wind a copper wire into its groove.
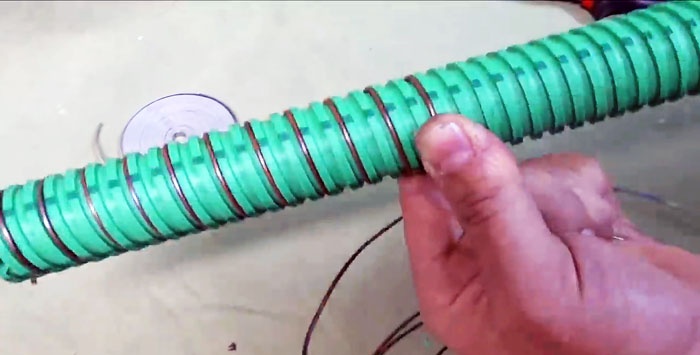
If you do not have such a tube, take a regular smooth one, but in this case the wire will have to be fixed periodically at a certain interval.
Then we wind galvanized tape into the gap between the copper.

These two tapes should not touch each other.
On one side we connect and draw a conclusion from the copper wire. And on the other side we make a tap from the zinc electrode.


We connect the wires and make terminals.

Install the element into the pipe.

We close the lid so that the tube on the lid goes inside the tube of the element with the electrodes.

We make an electrolyte: add a couple of tablespoons of soda to ordinary water. Next we fill it into the battery.


As you can see, the body is painted with black enamel. There is a valve on the side for releasing gases and draining liquid. Close with the second lid.
At this point, our chemical current source is ready.
The result of the salt battery
The result of the work is such that the open circuit voltage is 1.6 V. The short circuit current is 120 mA.
Now we connect the load. This is a single transistor boost converter for power supply LEDs.

LEDs shine brightly, consuming about 20 mA. As you can see, the drawdown was down to 1.2 V.

Next, let's try to power a 220 V lamp with a power of 3 W.

We also connect it through a converter.

It shines normally. The initial voltage drop was up to 0.8 V. After working for a couple of hours it was 0.6 V.
This battery will last for several hours. You can collect it and experiment with replacing the electrolyte, making it not from soda, but from ordinary table salt. Replace electrodes made of other metals. Who knows, maybe you can get more voltage and run time. Good luck!











